FDA/Registration Number
66-2-2-2-0006919

E1002060
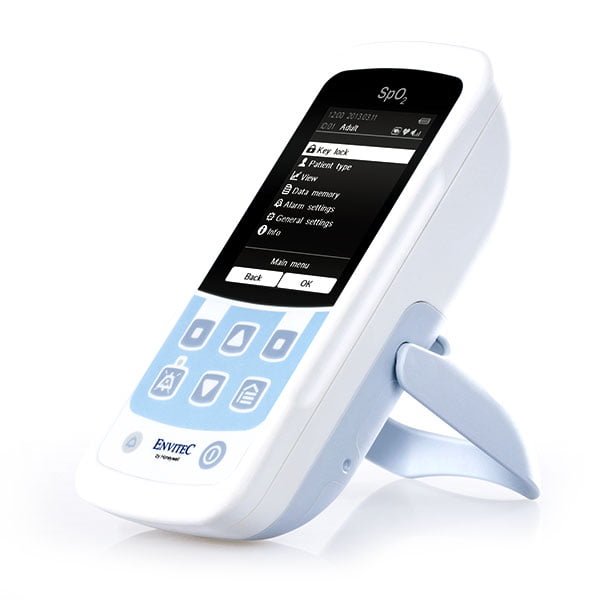
- Can adjust the display direction Can be used both vertically and horizontally.
- Backlit display, can read results in any direction.
- Can be connected to the base in many ways. Whether it’s a bed, an IV pole, or a table.
- Can connect and send data through PC Software.
- Durable Li-ion battery, rechargeable and reusable.

Precautions and Warnings
- A defective unit may not be used. Parts that are broken, worn out or contaminated must be replaced.
- The equipment should not be used adjacent to or stacked with other equipment and if adjacent or stacked use is necessary, the equipment should be observed to verify normal operation in the configuration in which it will be used.
- If the unit is not used for a prolonged period of time, the capacity of the battery must be checked before mobile use and charged first, if necessary
- Incorrect use or positioning of the sensors can result in measurement errors and can lead to constriction of body parts by the sensor cable, shearing off of skin portions by the sensor, etc.
- Exceeding the operating parameters or disregarding the measurement conditions will lead to incorrect measurements or, in severe case, damage to the MySign® S
- Do not damage or change the battery’s structure in any way
Notice the warnings on the label and accompanying documents before use
Distributed by
Alcotec International Co., Ltd
77/152 Sinn Sathorn Tower 35th Fl., Krungthonburi Road,
Klongtonsai, Klongsan, Bangkok 10600. Tel. 02-862-0959
ฆพ. 846/2568
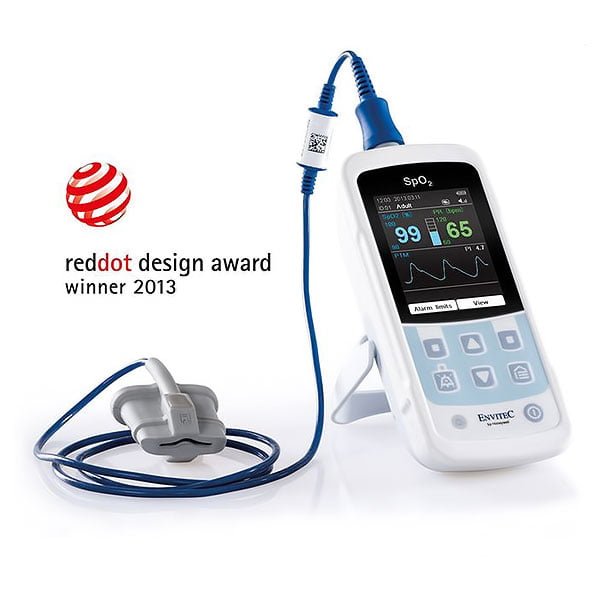
MySign® S Specification Sheet
RELATED PRODUCTS
Need more details for each of the product?
OUR CUSTOMERS









































