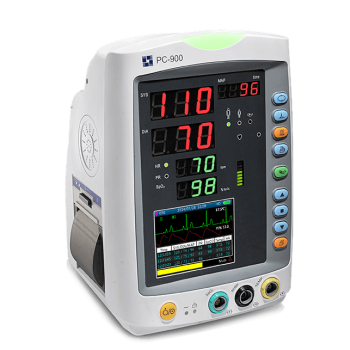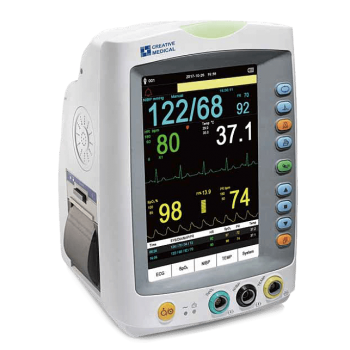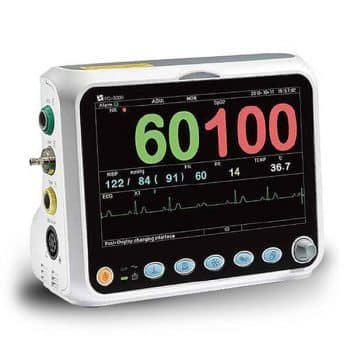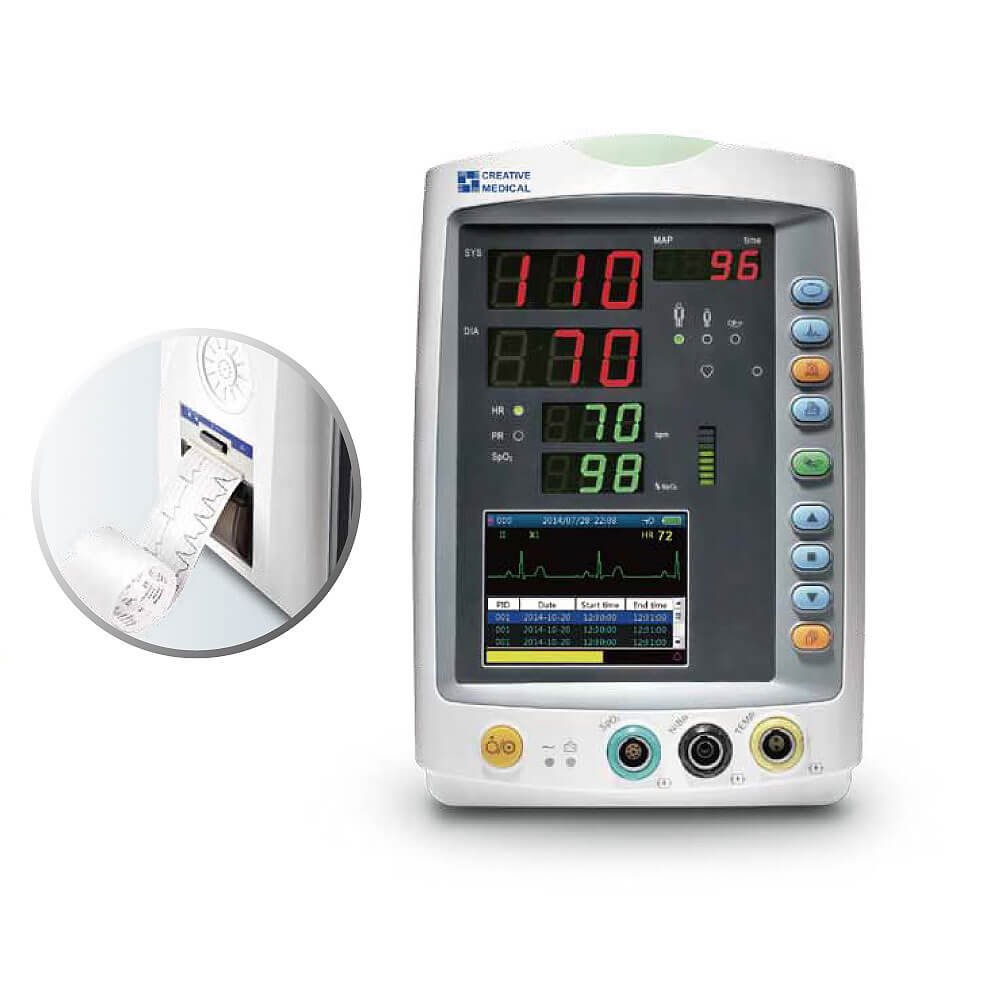
Vital Signs Monitor is a small multi-parameter patient monitor, which can monitor the vital physiological parameters: Non-Invasive Blood Pressure (NIBP), Pulse Oxygen Saturation (SpO2), Electrocardiogram (ECG), Temperature (TEMP). Economical and User Friendly.
Features
- 3.5 “ high resolution LCD display
- Table top design with user – friendly operation
- Up to I2000 groups NIBP and 2000 groups SpO2 storage
- Auto, Manual and STAT NIBP measuring mode
- Inbuilt rechargeable li-ion battery
- USB connectivity for data output and software upgrading
- Flexible configurations among SpO2, NIBP, TEMP and ECG
- Upgradable to Nellcor SpO2 and SunTech NIBP

Vital Sign Monitor PC900 PRO Specification Sheet
RELATED PRODUCTS
Need more details for each of the product?
OUR CUSTOMERS